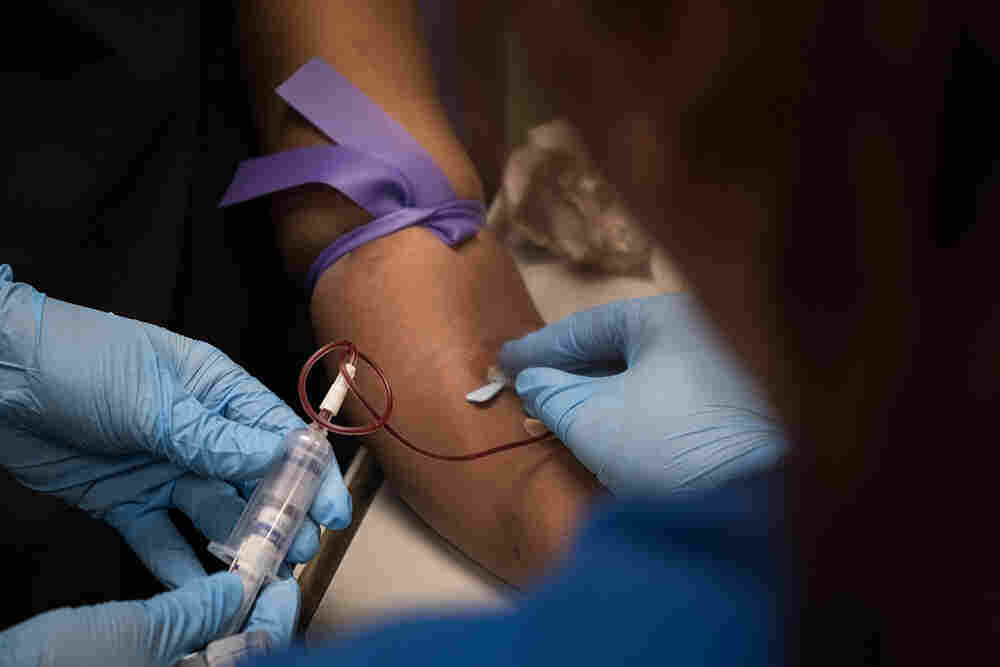
As part of a clinical trial to treat sickle cell disease, Victoria Gray (center) has vials of blood drawn by nurses Bonnie Carroll (left) and Kayla Jordan at TriStar Centennial Medical Center in Nashville, Tenn.
Meredith Rizzo/NPR
hide caption
toggle caption
Meredith Rizzo/NPR
Doctors are reporting the first evidence that genetically edited cells could offer a safe way to treat sickle cell disease, a devastating, incurable disorder that afflicts millions of peoplearound the world.
Billions of cells that were genetically modified with the powerful gene-editing technique called CRISPR have started working, as doctors had hoped, inside the body of the first sickle cell patient to receive the experimental treatment, according to highly anticipated data released Tuesday.
The edited cells are producing a crucial protein at levels that have already exceeded what doctors thought would be needed to alleviate the excruciating, life-threatening complications of the genetic blood disorder, the early data show. Moreover, the cells appear to have already started to spare the patient from the agonizing attacks of pain that are the hallmark of the disorder.
“We are very, very excited,” says Dr. Haydar Frangoul of the Sarah Cannon Research Institute in Nashville, Tenn., who is treating the patient. “This preliminary data shows for the first time that gene editing has actually helped a patient with sickle cell disease. This is definitely a huge deal.”
Frangoul and other researchers caution, however, that the results involve just one patient who was only recently treated. It is far too soon to answer the most crucial questions: Will the modified-cell treatment continue to improve the patient’s health? Will the treatment keep working? Will it help her live longer? Is it safe in the long term?
“We are hoping it is” a success, Frangoul says. But “it is still too early to celebrate.”
NPR has exclusive access to chronicle the experience of Frangoul’s patient, Victoria Gray of Forest, Miss., the first person with a genetic disease to be treated with CRISPR in the United States.
“So look at this,” Frangoul said recently with a smile, as he showed Gray her latest blood test results. The testing indicated that the genetically modified cells had already started producing the crucial protein at levels doctors hope will alleviate her suffering.
“I am super-excited about your results today,” Frangoul said.
In July, Gray was recovering from the medical procedure, which involved using an experimental technique called CRISPR to edit the genes of her own bone marrow cells.
Meredith Rizzo/NPR
hide caption
toggle caption
Meredith Rizzo/NPR
While Gray knows it’s still very early, she described how the treatment appears to be helping her. She has not suffered any of the painful attacks that torture sickle cell patients and has not needed to rush to the hospital for care since getting the modified cells this summer. She has not needed any blood transfusions either and has begun to reduce the pain medication she had been taking chronically.
“It’s a miracle,” says Gray, who says she has hope for the first time after a lifetime of struggling with excruciating pain and debilitating, life-threatening complications of the disease. Sickle cell disease is an inherited condition that is marked by defective oxygen-carrying red blood cells.
“When you pray for something for so long, all you can have is hope,” says Gray, 34, who has four children. “It’s amazing.”
The early results of the research were released by the two companies sponsoring the study that Gray volunteered for, Vertex Pharmaceuticals in Boston and CRISPR Therapeutics in Cambridge, Mass.
“This is a very important scientific and medical milestone,” said Dr. Jeffrey Leiden, chairman, president and CEO of Vertex. “We have potentially cured this patient with a single treatment. We are very hopeful.”
While Gray experienced some complications after the treatment, she recovered, and none of the problems is believed to have been caused by the treatment itself, according to the companies.
“I think it’s enormously exciting that we’ve reached a point where gene editing using CRISPR is being applied to sickle cell disease,” says Dr. Francis Collins, director of the National Institutes of Health. Collins, who is not involved in the research, noted that sickle cell disease affects about 100,000 people in the United States and millions more worldwide.
“To be able to take this new technology and give those people a chance for a new life, which it really would be, is a dream come true,” Collins says.
Because of the promise of research like this, the National Institutes of Health is launching a $200 million partnership with the Bill & Melinda Gates Foundation to find ways within 10 years to make expensive, complicated gene-based treatments affordable and practical in poor countries, where diseases such as sickle cell are most common. (The Gates Foundation provides support for coverage of global health and development by NPR.)
“The progress that we’ve seen in gene-therapy approaches for sickle cell disease in the U.S., including Victoria Gray and her involvement in this gene-editing protocol, made it clear that it was time to get started on the next phase of this,” Collins said. “If this is starting to work — but wouldn’t work where most of the patients are, which is Africa — we need to get busy and take it to the next level.”
The companies conducting the sickle cell study had previously only disclosed that the first patient in the trial had been treated and that another patient with a related blood disorder, beta thalassemia, had undergone CRISPR treatment this year and had not needed a blood transfusion for four months.
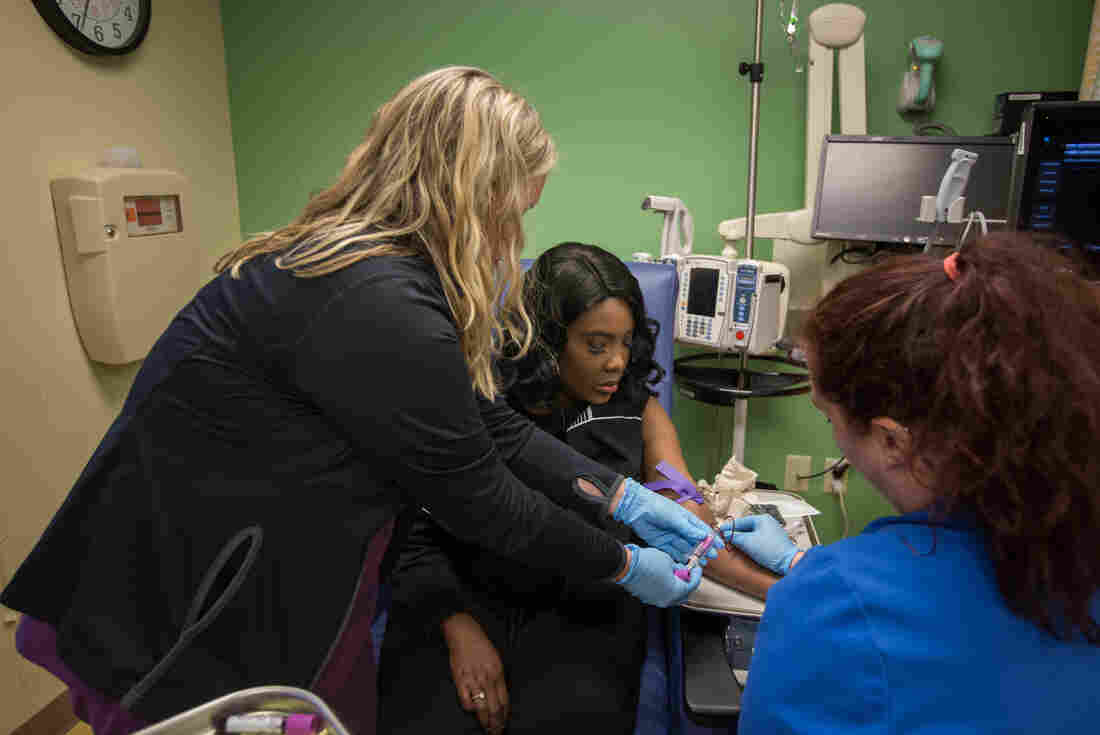
Gray underwent the gene-editing treatment at the Sarah Cannon Research Institute in Nashville, Tenn.
Meredith Rizzo/NPR
hide caption
toggle caption
Meredith Rizzo/NPR
That’s still true after nine months, according to the new data. The beta thalassemia patient, who was treated in Germany, has not been publicly identified. Typically, beta thalassemia patients need regular transfusions to survive. The CRISPR-treated patient normally needed more than 16 transfusions every year, according to the companies. That patient too experienced health problems after the treatment but also recovered, and none is believed to have been caused by the treatment.
“This is the first evidence that in people the new CRISPR technology has the potential to be curative for serious genetic diseases,” said Dr. David Altshuler, the chief scientific officer at Vertex.
“And this is just the beginning for this new type of therapy. Its applications can go beyond sickle cell disease and beta thalassemia to other genetic diseases.”
Many researchers think CRISPR could revolutionize medicine. The technique enables scientists to make very precise changes in DNA much more easily than ever before.
Doctors are also trying to use CRISPR to treat cancer. Most of that research is happening in China, and almost none of the results have been reported. But the University of Pennsylvania, which has tried CRISPR on three cancer patients, recently reported that gene editing appears feasible and safe. Another study recently started recruiting cancer patients in the U.S. and Australia.
Later this year, doctors in Boston are planning to use CRISPR for the first time to edit cells while they are still inside patients’ bodies — in retinas — in hopes of restoring vision in patients with an inherited form of blindness.
When NPR interviewed Gray most recently, she had just driven five hours back to Nashville after spending about a month at home in Mississippi with her family. She had been in Nashville for about three months over the summer to undergo the procedure on July 2 and then recover from the treatment, which required the equivalent of a bone marrow transplant.
During the return visit, Gray wore a black hooded sweatshirt emblazoned with the word “warrior” across the front.
“You know, they call sickle cell patients warriors, and I saw this shirt at Walmart so I had to get it,” Gray says. “It’s a constant battle.”
Sickle cell disease is a cruel genetic disorder that deforms red blood cells into defective sickle-shaped cells. The cells jam up the bloodstream, damaging vital organs and causing myriad health problems, in addition to the bouts of intense pain. Many patients with the disease can’t work or go to school. Many die before reaching middle age from complications such as heart attacks and strokes.
Researchers caution that Gray’s results are preliminary since they involve just one patient. There is still a lot that is unknown, including whether the treatment will make a lasting improvement in Gray’s health and whether it will be safe in the long term.
Meredith Rizzo/NPR
hide caption
toggle caption
Meredith Rizzo/NPR
“I had moments where I was just standing, laughing [and] talking with friends, and then the next thing you know, my husband had to carry me into the emergency room because I couldn’t use my legs because they hurt so bad,” says Gray, who has already suffered heart damage from her disease.
“And when you can’t help yourself, it’s just one of those things that just make you want to give up,” she says, her voice cracking with emotion.
Before Gray saw Frangoul, nurses at TriStar Centennial Medical Center took 16 vials of blood from her for tests as part of the clinical trial. The study is designed to eventually involve 45 patients in the United States, Canada and Europe. The beta thalassemia trial is designed to eventually involve 45 patients in Canada, Germany and London.
As the nurses filled one big blood tube after another, Gray described her homecoming a few weeks earlier.
“My oldest son — when he did his double take and realized I was in the car — he took off running, and he just grabbed me and held onto me. And the twins saw me from inside the house. My mama said that my daughter was, ‘My mama’s outside.’ She was just jumping. They knew it was Mama,” she says. “It’s emotional for me, you know, because I love them so much. I did this for them. So, it’s worth it.”
After Gray was done giving her blood sample, she met with Frangoul, who gave her a brief physical exam before showing her a sheet of paper with her latest test results.
“It looks like there are signs that you are starting to make fetal hemoglobin, which is very exciting,” Frangoul said.
Fetal hemoglobin is a protein that is normally produced only by fetuses and newborn babies for a short time after birth. So scientists used CRISPR to edit a gene in bone marrow cells that had been removed from Gray’s body.
The edited cells were infused back into her system, and the editing change allowed the cells to start producing fetal hemoglobin again. The hope is that the fetal hemoglobin will compensate for the genetic defect that has resulted in sickle cell disease and its abnormal form of adult hemoglobin.
The edited cells began functioning about a month after being infused into Gray’s body. Four months after Gray received the cells, her blood tests show that 46.6% of the hemoglobin in her system is fetal hemoglobin, according to the companies. That far exceeds the 20% to 30% that doctors thought would be needed to help her. And her fetal hemoglobin levels are still rising, Frangoul said in an interview. In addition, 94.7% of Gray’s red blood cells contain fetal hemoglobin, the companies reported.
Gray suffered a blood infection, gallstones and abdominal pain after the grueling procedure, which involved the equivalent of a bone marrow transplant. The beta thalassemia patient developed pneumonia and a liver problem. But none of those complications is believed to have been caused by the edited cells, the companies said.
Other researchers are testing another approach for sickle cell that involves using a virus to insert a healthy gene into sickle cell patients’ cells. That approach is also showing promise. Scientists are also planning to try to use CRISPR to correct the defective gene itself, which would be more difficult.
Frangoul stresses that it’s too soon to know if the fetal hemoglobin production will continue and how it might help Gray’s health over a longer period of time.
“I just want to make sure this is something we watch very carefully every visit and see how things are going,” he told Gray.
But Frangoul, medical director of pediatric hematology/oncology at HCA Healthcare’s TriStar Centennial Medical Center, knows Gray has been feeling better.
“You haven’t been in the hospital since I last saw you, correct?” he said. “No emergency rooms, no hospitals. How about that? That’s good. Excellent. Perfect. This is extremely encouraging.”
While Frangoul says some sickle cell patients can go for extended periods without severe attacks of pain, Gray says that normally she would have suffered some kind of episode in the period since she received the edited cells. In the two years before the treatment, Gray had experienced seven sickle cell crises a year.
At a recent checkup, Dr. Haydar Frangoul gave Gray preliminary results showing that her body is making fetal hemoglobin, a protein that could alleviate the symptoms of her sickle cell disease.
Meredith Rizzo/NPR
hide caption
toggle caption
Meredith Rizzo/NPR
“It’s special, especially coming up on the holidays, because sometimes I would be in the hospital on Christmas. And so I’m looking forward to a whole new life for all of us,” she said.
Gray calls edited cells her “supercells.”
“They seem to be super after all,” Gray said, laughing.
Frangoul plans to follow Gray for many more months to see if her “supercells” are really making her healthier, and for even longer to see if they help her live longer. Researchers plan to check up on Gray and other study subjects for 15 years to make sure the cells are not causing any long-term side effects of their own.
“This would be life-changing, not only for Victoria but for many sickle cell patients,” Frangoul said. “If this is determined to be safe and effective, I think it can be transformative for patients with sickle cell disease.”
Before the treatment, Gray was so weak from her disease that she couldn’t work or go to school and hadn’t been able to participate in many of her children’s activities.
Since the treatment, she felt strong enough to go to one of her son’s football games for the first time. She hopes that maybe now she’ll be able to spend a lot more time with her kids and see them grow up.
“I don’t really want anything extravagant,” Gray said. “I just want a simple life with my family and the people who I love and people that love me, and just live, you know? This could be the beginning of something special.”
Let’s block ads! (Why?)