2020 Affordable Care Act Health Plans: What’s New

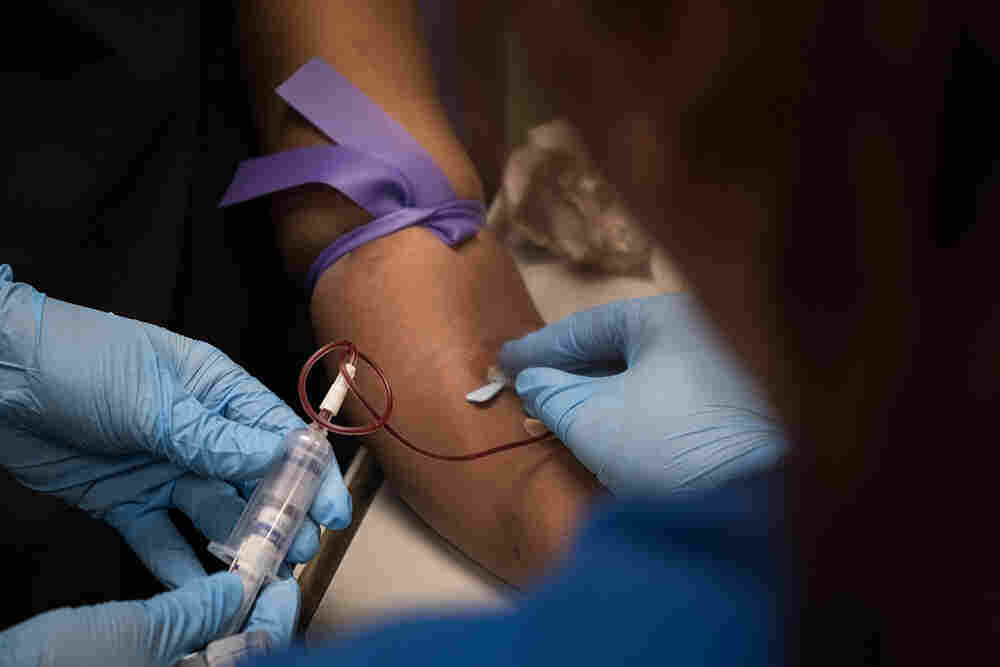
Enrollment help was plentiful for insurance sign-ups in the early years of the Affordable Care Act, such as at this clinic in Bear, Del., in 2014. Though the Trump administration has since slashed the outreach budget, about 930,000 people have signed up for ACA health plans so far this year.
Andrew Harrer/Bloomberg via Getty Images
hide caption
toggle caption
Andrew Harrer/Bloomberg via Getty Images
During Wednesday night’s Democratic presidential debate, candidates touched on “Medicare for All,” “Medicare for all who want it” and other ways to reform the American health system.
But in the backdrop, it’s once again sign-up season for Affordable Care Act health plans.
Despite repeated efforts by Republicans in Congress to undo the ACA, the controversial law’s seventh open-enrollment period launched earlier this month to relatively little fanfare. It ends Dec. 15.
Individual plans for 2020 are cheaper — premiums are lower, on average, and in some areas, people who qualify for government subsidies could end up with no monthly payment. Do check the fine print of your policy — in some cases, the patient’s share of other costs may have gone up.
Meanwhile, a pending court case threatens to overturn the entire law, with no clear replacement plan in the works. The result has been a cloud of confusion and misunderstanding — some even say misinformation — about the availability of this health coverage.
Here’s what consumers need to know.
The ACA is intact — at least for now.
The Affordable Care Act is still the law of the land.
The GOP-led Congress gutted a key part of the law — the penalty for the so-called individual mandate — that required everyone to have coverage. But other key tenets of the ACA remain in place, including the individual marketplace it created where people can shop for health coverage.
You might not hear much about that this year. The Trump administration has dramatically scaled back its outreach and marketing budget for open enrollment — allotting about $10 million for such efforts, compared with the more than $100 million the Obama administration spent.
Lack of outreach, combined with Republican efforts to overturn or undermine the law, could give consumers a wrong impression, says Katie Keith, a health policy consultant who frequently writes about the health law.
So far, about 930,000 people have signed up for coverage. That’s still slightly lower than where things were last year — and first-day Website glitches may have played a role, suggests Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms.
In week two of the sign-up period, enrollment was about 10% below what it was in last year’s second week – but the number of new customers has gone up.
“The irony is, things are really stable, and depending on your state, you can really save,” Keith says. “Without advertising and information, folks are going to miss out on the deals they could get.”
You can get a cheaper plan this year, but it will probably require a bit of work.
Those deals can be pretty significant. On average, premiums are down 4% nationally over last year for silver-level plans sold through the federal marketplace. In some states, they’re even cheaper.
Many people don’t realize that if their incomes are below 400% of the federal poverty level (just under $50,000 for an individual or about $103,000 for a family of four) they qualify for federal subsidies — tax credits that help them pay for individual marketplace plans, Corlette says. The federal government has an online calculator that indicates if you fit into this bracket.
That tax break can make it easier to find affordable, comprehensive coverage, Corlette says, and she recommends that people who already have 2019 ACA plans shop around the marketplace to make sure they get the best deal going into 2020. (What plans charge can change each year.)
People who bought coverage last fall and don’t shop around will be automatically reenrolled in those health plans — which may not be the best for their needs and may be more expensive than other options.
Also worth noting: Federal courts recently blocked a rule that would have penalized recent legal immigrants who use those subsidies. Known as the “public charge” rule, it would have counted that subsidy against people looking to stay longer in the United States.
The court ruling means that, at least for now, legal immigrants should also be able to purchase subsidized health insurance with no penalty, Corlette says.
“If you have concerns or are worried, you should consult an immigration attorney. But assuming you are a legal resident, nothing has changed in terms of your entitlement,” she says.
Not everything that looks like an ACA plan actually is one.
Consumers should be wary of plans that look like they meet ACA standards but actually fall short.
The Trump administration has loosened restrictions on non-ACA policies — “short-term plans” — so that they can last up to 12 months. (Previously they lasted only three months and were treated as bare-bones, stopgap insurance.)
Pitched as a cheaper alternative to ACA coverage, they are allowed to factor in preexisting medical conditions — and can deny insurance to people because of their medical history. They also generally cover a much narrower range of benefits. Some have lifetime caps on benefits, and they typically don’t cover prescription drugs.
These plans are not eligible for federal subsidies.
“If you were to Google something like ‘ACA plan’ or ‘Obamacare plan,’ the first results will return insurances that are not ACA coverage,” Corlette warned. “A lot of it is going to be junky, skimpy coverage.”
One easy way to distinguish ACA-compliant plans from others: Make sure you’re shopping through healthcare.gov or an alternative set up by some states. Consumers who work with an insurance broker should be sure to communicate their desire to choose an ACA-compliant plan.
Meanwhile, federal courts are weighing a legal challenge that could strike down the ACA.
It’s not clear what would happen if that attempt to overturn the federal health law succeeds. But, for now, the case shouldn’t affect your coverage decisions, all sides agree.
A group of Republican attorneys general and governors filed a lawsuit in 2018 that argues that since the Supreme Court upheld the ACA in 2012 specifically because its individual mandate was deemed a valid exercise of Congress’ taxing power, reducing that tax to zero — as Congress did — makes the entire law unconstitutional. It’s an argument that many legal experts say is shaky, but a federal judge in Texas agreed with those who brought the suit.
The case, known as Texas v. Azar, is awaiting a ruling from the 5th Circuit Court of Appeals. The Trump administration, meanwhile, has declined to defend the federal health law. A group of Democratic attorneys general has stepped in, in their stead.
If the appellate court sides with the trial judge to overturn the ACA, the decision would likely be stayed and the case appealed to the Supreme Court. That could drag things out until next summer at the earliest.
The administration hasn’t indicated what it might do if the health law is struck down — a scenario that would gut the individual marketplace and eliminate the ACA’s consumer protections. But most experts agree it is also not likely to affect the 2020 coverage year.
Earlier this month, Joe Grogan, who heads the White House’s Domestic Policy Council, reiterated that the administration “will be prepared after that decision comes down” — adding that “nothing’s going to happen immediately.”
“This will surely go to the Supreme Court,” Grogan said. “It may or may not be decided — probably not decided — before the election.”
But it’s unclear what a White House response would involve. Neither President Trump nor Republicans in Congress have put forth a health care proposal that would maintain the protections the Affordable Care Act put in place.
That could be worrisome down the line, Keith and Corlette say. But any impact is far enough away that it shouldn’t influence the way consumers choose health insurance for the next year.
“Nothing will change right away, and it shouldn’t affect anybody anytime soon,” Keith says. “Go enroll. Pay your premiums.”
Emmarie Huetteman contributed to this report.
Kaiser Health News is a nonprofit, editorially independent program of the Kaiser Family Foundation. KHN is not affiliated with Kaiser Permanente.
In ‘Canyon Dreams,’ A Navajo Town Struggles To Survive In An Often Hostile World
College and professional sports have a way of dominating the national headlines. But in some parts of America, high school athletics have become local obsessions.
In Pennsylvania, fans flock to school wrestling matches, while in Texas, high school football teams routinely sell out some of the state’s biggest stadiums.
In many parts of the western U.S., though, it’s the game of “rez ball” that has sports fans enchanted. As Michael Powell writes in his wonderful new book, Canyon Dreams, rez ball — so named because it’s played on Native American reservations — is a unique spin on basketball, “a quicksilver, sneaker-squeaking game of run, pass, pass, cut, and shoot, of spinning layups and quick shots and running, endless running … Play was swift and unrelenting as a monsoon-fed stream.”
And not many teams play it better than the Wildcats of Chinle High School, located in the Navajo Nation in northeastern Arizona. Powell, a New York Times columnist, spent a season in the small town of Chinle, watching the Wildcats take to the hardwood, and spending time with those who call the reservation home. Canyon Dreams is the product of his time in and around Chinle, and it’s a remarkable achievement.
Powell focuses heavily Raul Mendoza, the school’s “respected, although perhaps not beloved” basketball coach, whose career leading teams in the Southwest spanned decades: “He had lived a dozen lives in seventy years of wandering.” Mendoza is an old-school coach who’s determined to take Chinle to the state championships; he loves telling “corny jokes and he could name no rapper, past, present, or future.”
At the beginning of the season, Mendoza’s Chinle team has the potential to be a powerhouse, though you wouldn’t know it to look at them: Only three of the players on his team are over 6 ft. tall; the Wildcats’ point guard, Josiah Tsosie, stands at 5 ft. 4 in. Still, they’re scrappers in the rez ball tradition, which puts a premium on pugnacity and physicality: “Custom dictated that players help their opponents to their feet. They as quickly knocked them down again.”
Many of Mendoza’s players aren’t quite sure what to make of their laconic, reserved coach; some resent him for leaving them on the bench, while others don’t appreciate his often caustic assessments of their on-court performance. Despite his hard edges, though, Mendoza clearly cares for his players, especially those who have had to endure difficulty:
“Mendoza harbored special affection for the lost. … To take a well-adjusted kid and train and mold him was rewarding; to break through to the desperate, to give hope where there was none, was another mission entirely.”
And none of the players on the team have had easy lives. Chinle, like many nearby towns, has been beset by poverty, unemployment, domestic violence and alcoholism: “I knew of no player on Mendoza’s team whose family had not lost a relative to the bottle and fetal alcohol syndrome deformed some infants,” Powell writes.
He spends time with a wide array of people who live on the reservation, and presents their stories with a sympathy that’s never condescending. The results of his interviews can be heartbreaking: At one point, he takes a walk with a Navajo jeweler who used to play rez ball as a teenager; the next day, Powell learns that the man passed out the night before, the result of drinking too much, and froze to death.
Canyon Dreams is a book about basketball the same way that Buzz Bissinger’s Friday Night Lights is a book about football — while sports are the ostensible focus, Powell’s real interest is the community that drives the team. That’s not to say Powell’s coverage of Chinle’s games isn’t fascinating; indeed, he recaps the matches with an expert pacing, and creates an atmosphere of suspense as the Wildcats’ season progresses. He’s an excellent sportswriter with an obvious love for the game, and he does a great job explaining what makes rez ball so unique.
But it’s his deep dives into the lives of those associated with Chinle and its high school that sets Canyon Dreams apart. He profiles not just the players and coaching staff, but also teachers, townspeople and activists, and the result is a moving portrait of what it’s like to live on the reservation. Powell even incorporates memoir into the book, writing about his own explorations of the town, and how he came to be so invested in its people.
Canyon Dreams is difficult to categorize, but it’s unmistakably beautiful. Powell is a gifted and giving writer, and his book is at once a reflection on youth and ambition and a fascinating chronicle of a town’s struggle to survive in a world that’s often cruel and hostile. “Nothing about a basketball season is easy,” as Mendoza says. “Neither is life.”
Snarky Puppy: Tiny Desk Concert
Credit: Emily Bogle/NPR
Seconds before we hit record, Snarky Puppy‘s bandleader, Michael League leaned in to ask if he could “do a little crowd work.” I suspect he waited until the last second on purpose, but it’s been easy to trust this band when they have an idea, judging by the three Grammy Awards they get to dust off at home after every tour run.
What resulted was a Tiny Desk first: League divided the audience into two sections, one side clapping out a 3/4 beat and the other half a 4/4 beat, creating a polyrhythm that I’m sure a handful of coworkers didn’t feel so confident trying to pull off. But this band pulls you in with simple instruction and a little faith.
Snarky Puppy has been a force for a while now, earning the ears of millions for more than a decade. And their secret sauce? A long-simmered recipe of jazz, funk and gospel. The band started as college friends in the jazz program at the University of North Texas back in 2004. But the formative era came a few years later, after League picked up a few gospel gigs in Dallas and eventually brought the jazz students to church, where music plays a different role than it does in the classroom. In the pulpit, it’s a channel for spiritual healing, a communal experience between players and congregation. As an experiment, League pulled his jazz friends and his gospel bandmates into one ensemble, where the two groups bonded together and established ground-zero for building the sonic identity of Snarky Puppy.
Thirteen albums later, you can still hear these gospel and jazz orbits crashing into each other. They’re masters of theme and variation, offering anyone with a listening ear a place to grab hold. And people do. They’re a band whose lyric-less melodies are still yelled (sung back) to them at their concerts around the world, as a shared catharsis for everyone in the room.
SET LIST
- “Tarova”
- “Xavi”
MUSICIANS
Michael League: Bass; “JT” Thomas: drums; Nate Werth: percussion; Shaun Martin: keys; Bobby Sparks: keys; Justin Stanton: keys, trumpet; Jay Jennings: trumpet; Chris Bullock: saxophone, flute; Chris McQueen: guitar; Zach Brock: violin
CREDITS
Producers: Colin Marshall, Morgan Noelle Smith; Creative Director: Bob Boilen; Audio Engineer: Josh Rogosin; Videographers: Bronson Arcuri, Jack Corbett, CJ Riculan, Morgan Noelle Smith; Associate Producer: Bobby Carter Executive Producer: Lauren Onkey; VP, Programming: Anya Grundmann; Photo: Emily Bogle/NPR
10 Years After She Ran Across The U.S., Katie Visco Took On Australia
Long distance runner Katie Visco just completed a 2,200 mile, 119-day run across the Australian Outback.
RACHEL MARTIN, HOST:
A marathon is a little over 26 miles. Imagine running more than a marathon every single day for four months.
KATIE VISCO: I officially ran across Australia – 2,210 miles back roads, dirt roads, through the deserts with bicycle support.
NOEL KING, HOST:
That is Katie Visco. Her day job is delivering homemade soup on her bicycle. But this summer, she decided to run down the middle of Australia.
MARTIN: Katie ran between 28 and 38 miles every day while her husband, Henley, biked alongside her with their supplies. A lot of the terrain is desert, right? They dealt with heat, exhaustion and high desert winds.
VISCO: I could feel the wind emotionally because it’s so strong, in your face, right at you, and you can’t do anything about it. It’s literally like running through the most viscous sludge.
KING: The going got rough – like, really rough. In the sandiest stretch of desert, Henley had to push a supply bike for a few days. It weighed 350 pounds.
VISCO: And so with all of his might, he pushed, dragged, pulled this bike through two days of deep sand. There was a couple of moments where he sobbed. A huge lesson from the trip is that if I’m in pain – like, I have to run another 30 miles today and my foot hurts – whatever it is, it is brief.
MARTIN: Dragging yourself across the desert may not sound like fun, but Katie says she’s kind of sad it’s over.
VISCO: This run across Australia was ultimately a mission of self-discovery and self-love. I wanted to become somebody more than I thought I could be.
KING: So what is next for these two? Katie and her husband say they aren’t sure yet, but they are already daydreaming about it.
(SOUNDBITE OF MUSIC)
Copyright © 2019 NPR. All rights reserved. Visit our website terms of use and permissions pages at www.npr.org for further information.
NPR transcripts are created on a rush deadline by Verb8tm, Inc., an NPR contractor, and produced using a proprietary transcription process developed with NPR. This text may not be in its final form and may be updated or revised in the future. Accuracy and availability may vary. The authoritative record of NPR’s programming is the audio record.
America’s ‘Shame’: Medicaid Funding Slashed In U.S. Territories

Sandra King Young runs Medicaid in American Samoa, a U.S. territory that faces dramatic funding cuts to islanders’ health care unless Congress acts. “This is the United States’ shame in the islands,” she says.
Selena Simmons-Duffin/NPR
hide caption
toggle caption
Selena Simmons-Duffin/NPR
Right now, there are dozens of patients — U.S. citizens — in New Zealand hospitals who are fighting the clock. They have only a few weeks to recover and get home to the tiny island of American Samoa, a U.S. territory in the South Pacific.
“We have a cancer patient that is coming back in December,” says Sandra King Young, who runs the Medicaid program in American Samoa. “We can only give him six weeks of chemo, radiation and surgery. He has a good chance of survival if he has the full year of treatment, but not six weeks. The patient and family understand, and since they have no money, they have agreed to come back.”
The federal money to fully fund the Medicaid program in American Samoa and in all other U.S. territories is about to run out. As a consequence, the off-island referral program to treat conditions that the territory doesn’t have the local capacity or facilities to treat — the program that brought these patients to New Zealand — is getting shut down.
“It’s devastating for those people who need those lifesaving services,” King Young says. “People who need cancer treatment won’t get it. Children with rheumatic heart disease won’t get the heart surgeries that they need.”
All five of the U.S. territories affected — collectively home to more than 3 million Americans — are now desperately trying to figure out how to keep Medicaid running with only a fraction of the money they’ve had for the last several years. If Congress doesn’t increase the amount of designated money by the end of the year, the U.S. Virgin Islands and Guam say they would need to cut their Medicaid rolls in half; Puerto Rico says it would need to cut back dental and prescription drug services.
This is what people working on the issue have come to call the “Medicaid cliff.”
How did we get here?
When it comes to Medicaid, the federal government treats U.S. territories differently from how it treats states.
In U.S. states, the amount that the federal government contributes to Medicaid varies based on a formula in the law that relies on per capita income in each state. For instance, Alabama has a match rate of 72%; what this translates to is that for every dollar Alabama spends on Medicaid, the federal government contributes about $2.57 to the program.
But that’s not how it works in the territories, where even though the populations are all low income, the federal government’s match rate is set in statute at 55%. What this translates to is that for every dollar a U.S. territory spends on Medicaid, the federal government contributes $1.25.
The other significant difference is that the federal contribution to Medicaid in the territories is capped, with a set allotment of federal funds every year. Federal spending on Medicaid in states is not.
The low matching rate and the annual cap — that’s pretty much how it has always been for Medicaid in the territories, says Robin Rudowitz, who co-directs the Program on Medicaid and the Uninsured at the Kaiser Family Foundation. “These particular provisions historically have always been part of how they have been financed,” she says.
Here’s how the territories found themselves peering over the “Medicaid cliff.” For the last few years, the territories have had billions of dollars more in federal funding to run their programs than their usual capped allotments. First, the Affordable Care Act provided a one-time grant of $7.3 billion (which kicked off in 2011) for the territories’ Medicaid programs. Then, in 2017, after two Category 5 hurricanes ravaged Puerto Rico and the U.S. Virgin Islands, an extra $4.9 billion designated for their Medicaid programs was added to the 2018 Bipartisan Budget Act.
The Family Health Center Susana Centeno on remote Vieques island, part of Puerto Rico, was forced to close after it suffered damage from Hurricane Maria in 2017.
Xavier Garcia/Bloomberg/Getty Images
hide caption
toggle caption
Xavier Garcia/Bloomberg/Getty Images
Now, we’ve come to the cliff’s edge. Most of these two pots of money ran out at the end of September, with just a bit of funding from the ACA still available through the end of 2019.
That leaves, essentially, just the capped allotment of federal dollars for each of the territories — far less than what the territories have come to rely on to provide care to their Medicaid enrollees in recent years.
“The capped financing amounts were low and did not meet all of the needs of the population in the territories,” says Rudowitz. If the additional federal contributions are gone for good, she says, “given the share that they represent of the financing for Medicaid in these territories, it would mean major changes and reductions in care.”
The territories have known that this funding cliff was coming for years — the end to a major portion of the money was written into the ACA. Island health officials and care providers hoped Congress would have legislated a more permanent solution by now, but it hasn’t.
In recent months, Congress has passed continuing resolutions that have allowed the territories’ Medicaid programs to keep limping along. But without legislation that appropriates more money, the territories’ Medicaid programs all are in serious trouble.
The Medicaid and CHIP Payment and Access Commission, known as MACPAC, a nonpartisan government group that advises Congress on Medicaid policy, projected in July that all the territories will have budget shortfalls in 2020. When considered altogether, according to MACPAC’s report, their Medicaid programs will be more than $1 billion short.
This is why Medicaid directors in the U.S. territories are all sounding the alarm. King Young, of American Samoa, points to news coverage as far back as the 1960s that described her island as “America’s Shame in the South Seas” because of rampant pollution and neglect.
“Right now, this is the United States’ shame in the islands,” she says. “Tell me if that’s acceptable in the United States, to stand by and say, ‘Oh, sorry, we can’t give you that stent or that pacemaker, so it’s likely that you’ll have a stroke or heart attack and — that’s it.’ That’s what it means not to have enough Medicaid funding for the territories.”
“Full-court press” in Congress
The territories have been lobbying Congress, urging members to appropriate more funds for their Medicaid programs so health officials on the islands can avoid these hard choices — between, for instance, keeping a hospital going and paying for a patient’s cancer treatment.
Each territory has a nonvoting delegate in Congress. Puerto Rico also sent an additional delegation from the island last week to make the case to lawmakers that something must be done to stave off disaster.
“I would say that we’ve been doing a full-court press this entire calendar year,” says Jennifer Storipan, executive director of the Puerto Rico Federal Affairs Administration. “Gov. [Wanda] Vázquez has said that Medicaid funding is her highest priority for the island right now.”
A bill to temporarily increase funding to the territories’ Medicaid programs was passed by the House Energy and Commerce Committee in July but has yet to make it to the floor for a vote. It would greatly increase the federal match rate for Medicaid in all the territories and allocate a bonus $3 billion a year — but only for the next four years. There is no companion bill in the Senate.
“We’re in active negotiations with the Senate, but there is some opposition to our bill in the Senate,” says Rep. Darren Soto, D-Fla., who introduced the House legislation with Rep. Gus Bilirakis, R-Fla. Both represent districts in central Florida that have huge Puerto Rican populations, and Soto is of Puerto Rican descent. In the House, the bill has bipartisan support.
Opponents of the bill in the Senate say they worry that the billions of dollars that the territories are requesting could be misspent. Some senators have raised particular concerns about Puerto Rico. During a recent corruption probe, a Puerto Rican Medicaid official was arrested — that news emerged just a few days before a committee hearing on the territories’ Medicaid bill in July.
“So that didn’t help,” Soto says. Integrity provisions were added to the House bill, he says, specifically addressing concerns about Puerto Rico. “We do need to take it seriously and make sure that tax dollars are safeguarded,” he says, but adds, “There have been fraud instances in many states too, and we don’t take away their Medicaid funding.”
Some congressional Republicans note that the funding cliff was created by the Affordable Care Act, which gave extra funding to the territories for a limited time. In a letter to Secretary of Health and Human Services Alex Azar in July, several Republican members of the Senate Finance Committee raised concerns about Puerto Rico’s Medicaid spending.
“We are again confronting proposals for what amounts to another extension of boosted funding with no permanence or certainty and without any resolution of the Medicaid funding cliff constructed as part of the ACA,” they wrote.
“It is true: Giving these additional funds with a set expiration date, the legislation itself did create the cliff,” acknowledges the Kaiser Family Foundation’s Rudowitz. However, she says, Congress now should consider “the implications of letting that cliff happen and what that means to the health care systems in these territories.”
Planning for the worst
Medicaid program administrators from each of the territories gathered in an impromptu meeting outside the annual Medicaid directors conference last week. They have spent a lot of time together in Washington recently, testifying before congressional committees about the Medicaid funding crisis as it approaches.
“We were here in May. We were here in June,” Michal Rhymer-Browne, assistant commissioner with the Virgin Islands’ Department of Human Services, told NPR that day. “We pleaded. We shared ourselves — everything that we could.”
Despite their pleas, Congress has yet to act.
There are three options for managing a slashed budget for a program like Medicaid: reduce the size of payments to providers, cut the rolls or cover fewer services. All the territories say there’s no way to go lower on provider payments — Puerto Rico’s struggle with provider flight because of low pay is well known. So cutting the rolls and covered services is where the programs would need to find cuts, representatives of the territories say.
“Guam and [the Northern Mariana Islands] are about to topple over the cliff,” Rhymer-Browne says. “And for the Virgin Islands, we are nearing the cliff and seeing the bottom right now.”
Many of the territories would like to make improvements in the care they provide — invest in better facilities, recruit great health care providers, improve preventive care. All of that comes with upfront costs, they say, and they’ve had so much budget uncertainty that it has been impossible to move forward with those kinds of projects.
Right now, they’re just trying to keep the lights on.
“The urgency is here for us to let our congressional members know — even going to January is extremely dangerous,” Rhymer-Browne says. “It’s already catastrophic for our territories. We really need them to make a move.”
Want New Taxes To Pay For Health Care? Lessons From The Affordable Care Act

A demonstrator celebrated outside the U.S. Supreme Court in 2015 after the court voted to uphold key tax subsidies that are part of the Affordable Care Act. But federal taxes and other measures designed to pay for the health care the ACA provides have not fared as well.
Andrew Harrer/Bloomberg via Getty Images
hide caption
toggle caption
Andrew Harrer/Bloomberg via Getty Images
It was a moment of genuine bipartisanship at the House Ways and Means Committee in October, as Democratic and Republican sponsors alike praised a bill called the “Restoring Access to Medication Act of 2019.”
The bill, approved by the panel on a voice vote, would allow consumers to use their tax-free flexible spending accounts or health savings accounts to pay for over-the-counter medications and women’s menstrual products.
Assuming the measure ultimately finds its way into law, it would also represent the latest piece of the Affordable Care Act’s financing to be undone.
Over-the-counter medication had been eligible to be considered a pretax expenditure in this way, before the ACA. But that eligibility was eliminated as part of a long list of new taxes and other measures that were designed to generate revenue to help pay for expanding health coverage to more people — a roughly $1 trillion cost of the health law over its first 10 years.
“It is paid for. It is fiscally responsible,” said President Barack Obama as he signed the ACA into law in 2010.
But not so much anymore. Many of the taxes and other provisions aimed at paying for that expanded health coverage “have been eliminated, delayed or are in jeopardy,” says Marc Goldwein of the Committee for a Responsible Federal Budget, a nonpartisan budget watchdog group. “All this stuff, it turns out, is very unpopular.”
The first piece of financing to disappear happened before most of the law even took effect. In 2011, Congress repealed a requirement that small businesses report to the IRS any payment of more than $600 to a vendor. The idea was that if more such payments were reported to taxing authorities, more taxes due on that income would actually get paid.
But small businesses complained — loudly — that the new paperwork requirement would be excessive, and Congress (and Obama) eventually agreed. The change alone eliminated an estimated $17 billion in ACA financing over 10 years.
Next, in 2015, Congress delayed (for the first time) the “Cadillac tax,” a 40% tax on the most generous employer health plans; one goal of that tax had been to curb excessive use of medical services.
That congressional delay came after intense lobbying by a coalition of business, labor and patient advocacy groups that banded together in a group called the Alliance to Fight the 40. It first got Congress to delay the Cadillac tax’s implementation from 2018 to 2020, then further pushed that date to 2022. And this past summer the House voted overwhelmingly to altogether eliminate the tax, which had been estimated to raise nearly $200 billion over the next decade.
Also on ice, thanks to that 2018 bill, are levies that were supposed to be paid by medical-device makers and health insurance companies, originally worth a combined $80 billion in financing during the law’s first decade.
Yet another — albeit fairly small — source of financing for the law went away in the 2017 GOP tax bill, which zeroed out the tax penalty for failing to have health insurance. The penalty raised $4 billion in 2018, the last year it was in effect.
Now it should be pointed out that the two ACA taxes that generate the most revenue are still on the books and collecting money. They are aimed at people with high incomes (more than $200,000 for individuals and $250,000 for couples) and were estimated to bring in more than $200 billion from 2010 to 2019. The measures, which don’t deal directly with services or provisions of the ACA, raise Medicare taxes on people at those higher incomes and increase taxes on unearned income.
The durability of those two taxes does not surprise Goldwein. Some are “unpopular to repeal,” he says, like “a tax on the rich that funds Medicare.”
What Goldwein does find surprising, though, is how durable some of the ACA’s other financing measures — reductions in spending — have been. For example, the health law, somewhat controversially, reduced Medicare payments to hospitals, insurance companies and a broad array of other health providers.
“The Medicare cuts have been for the most part surprisingly sustainable politically,” Goldwein says. Even when the GOP took over the House in 2011, its budget maintained the reductions from the ACA. So did the 2017 GOP “repeal and replace” proposal.
On the other hand, the appointed board of experts that was to rein in future Medicare spending, the “Independent Payment Advisory Board,” never got off the ground. Congress formally repealed it in 2018.
So what does this all mean? The past decade has shown that it has been relatively easy to make hard-won tax increases go away, suggesting that interest groups — particularly health industry groups representing drugmakers, insurers and hospitals — still wield a lot of power on Capitol Hill.
“Right now, everyone wants to cancel a 3% tax on the health insurance industry,” he says, referring to the current efforts of a major ad campaign by a coalition of small-business owners and insurance groups to get Congress to delay or cancel that ACA-linked tax.
Given how much money from health insurers is going into fighting that tax, he says, how likely is it that Congress — even one controlled by Democrats — would really “cancel the whole industry” by passing a “Medicare for All” bill?
Kaiser Health News is a nonprofit, editorially independent program of the Kaiser Family Foundation. KHN is not affiliated with Kaiser Permanente.
Gene-Edited ‘Supercells’ Make Progress In Fight Against Sickle Cell Disease
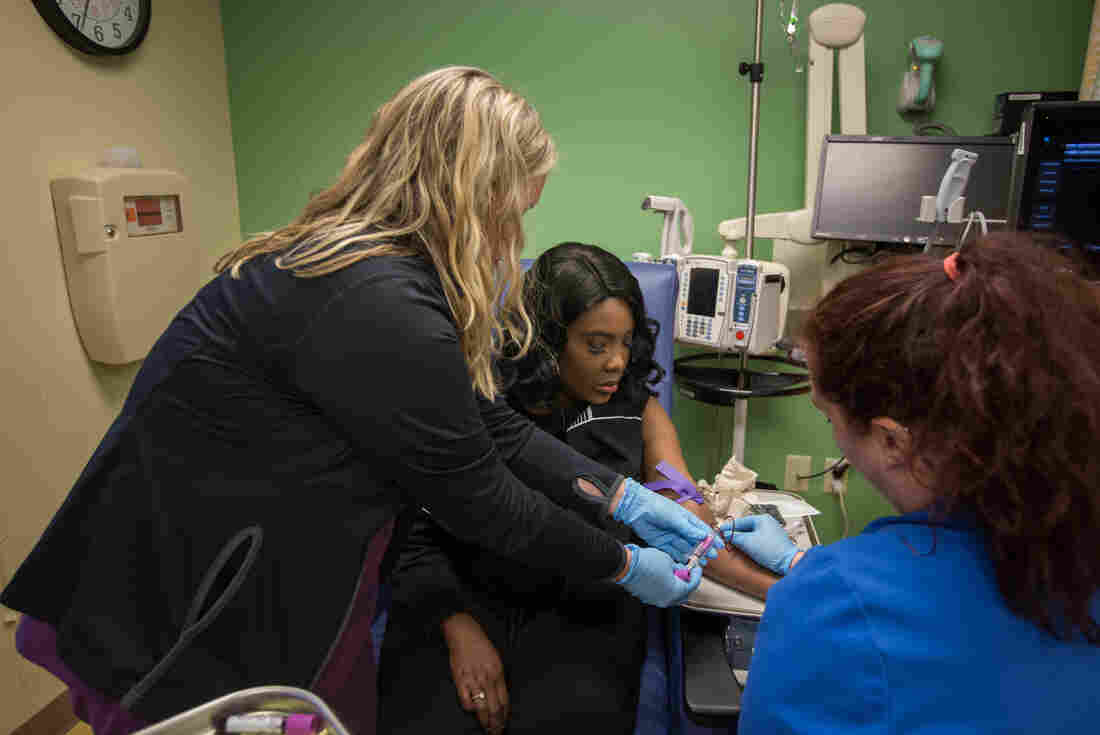
As part of a clinical trial to treat sickle cell disease, Victoria Gray (center) has vials of blood drawn by nurses Bonnie Carroll (left) and Kayla Jordan at TriStar Centennial Medical Center in Nashville, Tenn.
Meredith Rizzo/NPR
hide caption
toggle caption
Meredith Rizzo/NPR
Doctors are reporting the first evidence that genetically edited cells could offer a safe way to treat sickle cell disease, a devastating, incurable disorder that afflicts millions of peoplearound the world.
Billions of cells that were genetically modified with the powerful gene-editing technique called CRISPR have started working, as doctors had hoped, inside the body of the first sickle cell patient to receive the experimental treatment, according to highly anticipated data released Tuesday.
The edited cells are producing a crucial protein at levels that have already exceeded what doctors thought would be needed to alleviate the excruciating, life-threatening complications of the genetic blood disorder, the early data show. Moreover, the cells appear to have already started to spare the patient from the agonizing attacks of pain that are the hallmark of the disorder.
“We are very, very excited,” says Dr. Haydar Frangoul of the Sarah Cannon Research Institute in Nashville, Tenn., who is treating the patient. “This preliminary data shows for the first time that gene editing has actually helped a patient with sickle cell disease. This is definitely a huge deal.”
Frangoul and other researchers caution, however, that the results involve just one patient who was only recently treated. It is far too soon to answer the most crucial questions: Will the modified-cell treatment continue to improve the patient’s health? Will the treatment keep working? Will it help her live longer? Is it safe in the long term?
“We are hoping it is” a success, Frangoul says. But “it is still too early to celebrate.”
NPR has exclusive access to chronicle the experience of Frangoul’s patient, Victoria Gray of Forest, Miss., the first person with a genetic disease to be treated with CRISPR in the United States.
“So look at this,” Frangoul said recently with a smile, as he showed Gray her latest blood test results. The testing indicated that the genetically modified cells had already started producing the crucial protein at levels doctors hope will alleviate her suffering.
“I am super-excited about your results today,” Frangoul said.
In July, Gray was recovering from the medical procedure, which involved using an experimental technique called CRISPR to edit the genes of her own bone marrow cells.
Meredith Rizzo/NPR
hide caption
toggle caption
Meredith Rizzo/NPR
While Gray knows it’s still very early, she described how the treatment appears to be helping her. She has not suffered any of the painful attacks that torture sickle cell patients and has not needed to rush to the hospital for care since getting the modified cells this summer. She has not needed any blood transfusions either and has begun to reduce the pain medication she had been taking chronically.
“It’s a miracle,” says Gray, who says she has hope for the first time after a lifetime of struggling with excruciating pain and debilitating, life-threatening complications of the disease. Sickle cell disease is an inherited condition that is marked by defective oxygen-carrying red blood cells.
“When you pray for something for so long, all you can have is hope,” says Gray, 34, who has four children. “It’s amazing.”
The early results of the research were released by the two companies sponsoring the study that Gray volunteered for, Vertex Pharmaceuticals in Boston and CRISPR Therapeutics in Cambridge, Mass.
“This is a very important scientific and medical milestone,” said Dr. Jeffrey Leiden, chairman, president and CEO of Vertex. “We have potentially cured this patient with a single treatment. We are very hopeful.”
While Gray experienced some complications after the treatment, she recovered, and none of the problems is believed to have been caused by the treatment itself, according to the companies.
“I think it’s enormously exciting that we’ve reached a point where gene editing using CRISPR is being applied to sickle cell disease,” says Dr. Francis Collins, director of the National Institutes of Health. Collins, who is not involved in the research, noted that sickle cell disease affects about 100,000 people in the United States and millions more worldwide.
“To be able to take this new technology and give those people a chance for a new life, which it really would be, is a dream come true,” Collins says.
Because of the promise of research like this, the National Institutes of Health is launching a $200 million partnership with the Bill & Melinda Gates Foundation to find ways within 10 years to make expensive, complicated gene-based treatments affordable and practical in poor countries, where diseases such as sickle cell are most common. (The Gates Foundation provides support for coverage of global health and development by NPR.)
“The progress that we’ve seen in gene-therapy approaches for sickle cell disease in the U.S., including Victoria Gray and her involvement in this gene-editing protocol, made it clear that it was time to get started on the next phase of this,” Collins said. “If this is starting to work — but wouldn’t work where most of the patients are, which is Africa — we need to get busy and take it to the next level.”
The companies conducting the sickle cell study had previously only disclosed that the first patient in the trial had been treated and that another patient with a related blood disorder, beta thalassemia, had undergone CRISPR treatment this year and had not needed a blood transfusion for four months.
Gray underwent the gene-editing treatment at the Sarah Cannon Research Institute in Nashville, Tenn.
Meredith Rizzo/NPR
hide caption
toggle caption
Meredith Rizzo/NPR
That’s still true after nine months, according to the new data. The beta thalassemia patient, who was treated in Germany, has not been publicly identified. Typically, beta thalassemia patients need regular transfusions to survive. The CRISPR-treated patient normally needed more than 16 transfusions every year, according to the companies. That patient too experienced health problems after the treatment but also recovered, and none is believed to have been caused by the treatment.
“This is the first evidence that in people the new CRISPR technology has the potential to be curative for serious genetic diseases,” said Dr. David Altshuler, the chief scientific officer at Vertex.
“And this is just the beginning for this new type of therapy. Its applications can go beyond sickle cell disease and beta thalassemia to other genetic diseases.”
Many researchers think CRISPR could revolutionize medicine. The technique enables scientists to make very precise changes in DNA much more easily than ever before.
Doctors are also trying to use CRISPR to treat cancer. Most of that research is happening in China, and almost none of the results have been reported. But the University of Pennsylvania, which has tried CRISPR on three cancer patients, recently reported that gene editing appears feasible and safe. Another study recently started recruiting cancer patients in the U.S. and Australia.
Later this year, doctors in Boston are planning to use CRISPR for the first time to edit cells while they are still inside patients’ bodies — in retinas — in hopes of restoring vision in patients with an inherited form of blindness.
When NPR interviewed Gray most recently, she had just driven five hours back to Nashville after spending about a month at home in Mississippi with her family. She had been in Nashville for about three months over the summer to undergo the procedure on July 2 and then recover from the treatment, which required the equivalent of a bone marrow transplant.
During the return visit, Gray wore a black hooded sweatshirt emblazoned with the word “warrior” across the front.
“You know, they call sickle cell patients warriors, and I saw this shirt at Walmart so I had to get it,” Gray says. “It’s a constant battle.”
Sickle cell disease is a cruel genetic disorder that deforms red blood cells into defective sickle-shaped cells. The cells jam up the bloodstream, damaging vital organs and causing myriad health problems, in addition to the bouts of intense pain. Many patients with the disease can’t work or go to school. Many die before reaching middle age from complications such as heart attacks and strokes.
Researchers caution that Gray’s results are preliminary since they involve just one patient. There is still a lot that is unknown, including whether the treatment will make a lasting improvement in Gray’s health and whether it will be safe in the long term.
Meredith Rizzo/NPR
hide caption
toggle caption
Meredith Rizzo/NPR
“I had moments where I was just standing, laughing [and] talking with friends, and then the next thing you know, my husband had to carry me into the emergency room because I couldn’t use my legs because they hurt so bad,” says Gray, who has already suffered heart damage from her disease.
“And when you can’t help yourself, it’s just one of those things that just make you want to give up,” she says, her voice cracking with emotion.
Before Gray saw Frangoul, nurses at TriStar Centennial Medical Center took 16 vials of blood from her for tests as part of the clinical trial. The study is designed to eventually involve 45 patients in the United States, Canada and Europe. The beta thalassemia trial is designed to eventually involve 45 patients in Canada, Germany and London.
As the nurses filled one big blood tube after another, Gray described her homecoming a few weeks earlier.
“My oldest son — when he did his double take and realized I was in the car — he took off running, and he just grabbed me and held onto me. And the twins saw me from inside the house. My mama said that my daughter was, ‘My mama’s outside.’ She was just jumping. They knew it was Mama,” she says. “It’s emotional for me, you know, because I love them so much. I did this for them. So, it’s worth it.”
After Gray was done giving her blood sample, she met with Frangoul, who gave her a brief physical exam before showing her a sheet of paper with her latest test results.
“It looks like there are signs that you are starting to make fetal hemoglobin, which is very exciting,” Frangoul said.
Fetal hemoglobin is a protein that is normally produced only by fetuses and newborn babies for a short time after birth. So scientists used CRISPR to edit a gene in bone marrow cells that had been removed from Gray’s body.
The edited cells were infused back into her system, and the editing change allowed the cells to start producing fetal hemoglobin again. The hope is that the fetal hemoglobin will compensate for the genetic defect that has resulted in sickle cell disease and its abnormal form of adult hemoglobin.
The edited cells began functioning about a month after being infused into Gray’s body. Four months after Gray received the cells, her blood tests show that 46.6% of the hemoglobin in her system is fetal hemoglobin, according to the companies. That far exceeds the 20% to 30% that doctors thought would be needed to help her. And her fetal hemoglobin levels are still rising, Frangoul said in an interview. In addition, 94.7% of Gray’s red blood cells contain fetal hemoglobin, the companies reported.
Gray suffered a blood infection, gallstones and abdominal pain after the grueling procedure, which involved the equivalent of a bone marrow transplant. The beta thalassemia patient developed pneumonia and a liver problem. But none of those complications is believed to have been caused by the edited cells, the companies said.
Other researchers are testing another approach for sickle cell that involves using a virus to insert a healthy gene into sickle cell patients’ cells. That approach is also showing promise. Scientists are also planning to try to use CRISPR to correct the defective gene itself, which would be more difficult.
Frangoul stresses that it’s too soon to know if the fetal hemoglobin production will continue and how it might help Gray’s health over a longer period of time.
“I just want to make sure this is something we watch very carefully every visit and see how things are going,” he told Gray.
But Frangoul, medical director of pediatric hematology/oncology at HCA Healthcare’s TriStar Centennial Medical Center, knows Gray has been feeling better.
“You haven’t been in the hospital since I last saw you, correct?” he said. “No emergency rooms, no hospitals. How about that? That’s good. Excellent. Perfect. This is extremely encouraging.”
While Frangoul says some sickle cell patients can go for extended periods without severe attacks of pain, Gray says that normally she would have suffered some kind of episode in the period since she received the edited cells. In the two years before the treatment, Gray had experienced seven sickle cell crises a year.
At a recent checkup, Dr. Haydar Frangoul gave Gray preliminary results showing that her body is making fetal hemoglobin, a protein that could alleviate the symptoms of her sickle cell disease.
Meredith Rizzo/NPR
hide caption
toggle caption
Meredith Rizzo/NPR
“It’s special, especially coming up on the holidays, because sometimes I would be in the hospital on Christmas. And so I’m looking forward to a whole new life for all of us,” she said.
Gray calls edited cells her “supercells.”
“They seem to be super after all,” Gray said, laughing.
Frangoul plans to follow Gray for many more months to see if her “supercells” are really making her healthier, and for even longer to see if they help her live longer. Researchers plan to check up on Gray and other study subjects for 15 years to make sure the cells are not causing any long-term side effects of their own.
“This would be life-changing, not only for Victoria but for many sickle cell patients,” Frangoul said. “If this is determined to be safe and effective, I think it can be transformative for patients with sickle cell disease.”
Before the treatment, Gray was so weak from her disease that she couldn’t work or go to school and hadn’t been able to participate in many of her children’s activities.
Since the treatment, she felt strong enough to go to one of her son’s football games for the first time. She hopes that maybe now she’ll be able to spend a lot more time with her kids and see them grow up.
“I don’t really want anything extravagant,” Gray said. “I just want a simple life with my family and the people who I love and people that love me, and just live, you know? This could be the beginning of something special.”
Open Enrollment For 2020 Obamacare Has Begun
This year the federal health insurance marketplace Healthcare.gov has a few new bells and whistles. (This piece initially aired on Nov. 3, 2019 on Weekend Edition Sunday.)
When It Comes To Vaping, Health Officials Insist There’s A Lot At Stake
A former vaper has a warning for others. And, scientists work to understand how nicotine affects the teenage brain. (This segment initially aired on Oct. 10, 2019 on Morning Edition.)
Trump Administration’s Efforts To Ban Most Flavored Vaping Products Have Stalled Out
The White House is apparently backpedaling on its plan to ban most flavors in vaping products. The proposed FDA rule is unpopular with vape shop owners, and that’s creating political blowback.
ARI SHAPIRO, HOST:
The Trump administration’s efforts to ban most flavored vaping products have stalled out. The president announced two months ago that he would do something to address the youth vaping epidemic. A plan was supposed to have been announced in a matter of weeks. NPR science correspondent Richard Harris explains what happened instead.
RICHARD HARRIS, BYLINE: When President Trump said he was endorsing a Food and Drug Administration proposal to ban most flavored vaping products, he acknowledged there were some economic consequences.
(SOUNDBITE OF ARCHIVED RECORDING)
PRESIDENT DONALD TRUMP: Vaping has become a very big business, as I understand it – like, a giant business in a very short period of time. But we can’t allow people to get sick, and we can’t have our youth be so affected.
HARRIS: The policy proposal hit just as health officials were investigating lung injuries and deaths among people who vaped. Scientists now say that’s primarily from vaping dubious marijuana products. But Paul Billings at the American Lung Association was also focused on the role that flavored e-cigarettes played in teen nicotine addiction.
PAUL BILLINGS: We were very optimistic, encouraged when the president announced that he wanted to clear the markets of all flavored e-cigarettes that play such an important role in addicting millions of kids to these products.
HARRIS: That optimism started to fade after the policy did not appear as promised in the following weeks.
BILLINGS: It stretched into months. A package was sent to the White House for review, and then it cleared. And then everything stopped on November 5.
HARRIS: The Washington Post reports that’s when the president’s political staff advised him not to sign off on the new rules.
Paul Blair at the conservative group Americans for Tax Reform was part of the push against the new rules.
PAUL BLAIR: Look. There are legitimate concerns about teens experimenting with these products, but running towards the 1920s in terms of prohibition is a vote-losing issue.
HARRIS: That message hit the airwaves of Fox News, which ran commercials like this one.
(SOUNDBITE OF ARCHIVED RECORDING)
UNIDENTIFIED PERSON #1: If you enact a flavor ban, this will cost you the election.
UNIDENTIFIED PERSON #2: I vape, and I vote.
UNIDENTIFIED PERSON #3: Vapor Technology Association is responsible for the content of this advertising.
HARRIS: And advocates assert that a vaping flavor ban could tilt the election in close states against Trump. Blair’s organization polled people who vape in swing states like Michigan a few years back.
BLAIR: Three out of 4 of these adult consumers are single-issue voters.
HARRIS: And Blair says that issue is access to vaping, including flavored products. Some also argue that getting rid of flavored vaping products could drive people back to smoking cigarettes, which are the leading preventable cause of death in the United States. On top of that, Blair says the industry itself provides 150,000 jobs through vape shops and manufacturers.
BLAIR: It’d be a pretty significant hit in an election year for a guy that’s focused on deregulations, spurring economic growth and not killing jobs.
HARRIS: Big Tobacco is also part of the story, says Paul Billings at the American Lung Association.
BILLINGS: The largest tobacco companies in the world, like Altria and Reynolds, are major players in the e-cigarette business, along with these vape shops.
HARRIS: And these forces appear to have won out over the public health advocates. So Billings says the lead could well shift to states, counties and cities.
BILLINGS: And so we fully expect, irrespective of what the administration does or does not do, that states and localities will continue to move forward.
HARRIS: A White House spokesman says the new rules haven’t been killed, but it’s not clear what, if anything, will survive this process.
Richard Harris, NPR News.
(SOUNDBITE OF SMALL BLACK SONG, “SOPHIE”)
Copyright © 2019 NPR. All rights reserved. Visit our website terms of use and permissions pages at www.npr.org for further information.
NPR transcripts are created on a rush deadline by Verb8tm, Inc., an NPR contractor, and produced using a proprietary transcription process developed with NPR. This text may not be in its final form and may be updated or revised in the future. Accuracy and availability may vary. The authoritative record of NPR’s programming is the audio record.